Evaluating the Microbial Risk of Inclusions in a Food Formulation

Using inclusions in a food formulation can take a product from good to over the top great. Products with inclusions not only grab your attention visually, but also have added flavor and texture to an otherwise boring base formulation. There are the classic baked good products with inclusions like blueberry muffins and chocolate chip cookies, but new inclusions like colorful confetti pieces, honeycomb crunches, and freeze dried vegetables or proteins are driving innovation. The choices for inclusions are endless creating a sensory revolution unlike any we have experienced in the food industry.
Although inclusions open the door to an exciting new area in product development, they can present a challenge when validating a microbial kill step for food safety. The time to target temperature (come-up time) for an inclusion during processing may be different than the base formulation which can have an effect on pathogen lethality. Secondly, inclusions are typically very different in matrix composition when compared to the base formulation they are immersed in. Inclusions with high fat content and low moisture can have a negative impact on inactivation of pathogens such as Salmonella spp. in thermal process validation trials. Lastly, monitoring inclusion internal temperature is not always possible due to the composition and size of the inclusion. Inclusions millimeters in size are too small to embed a thermocouple for modeling temperature, but these small pieces can be a universe of safe harborage for microorganisms.
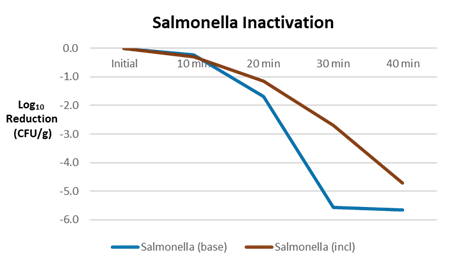
An example comparing the difference in Salmonella spp. lethality in the base formulation vs. the inclusion is shown below for a pet food biscuit formulation with freeze dried peas and carrots. As shown, inactivation of Salmonella spp. for the inoculated inclusions lags inactivation in the inoculated base formulation with a marked difference after 30 minutes of bake time. The base formulation demonstrates a greater than 5 log reduction vs. the inclusion with an approximate 3 log reduction at the same time.
So how can we be sure we are achieving the desired inactivation of pathogens in formulations containing inclusions? How can we gain information on the risk of our inclusions? Will we need to perform multiple challenge studies to understand the risk of inclusions?
The good news is, information on Salmonella spp. inactivation in both the base formulation and in the inclusion can be accomplished in one trial with creative study protocol design. For studies in which thermal processing can be reproduced in the lab (such as baking), the inclusion can be inoculated with Salmonella spp. and the base with a Salmonella surrogate organism representing Salmonella, such as E. faecium. Post thermal processing, quantification of the two organisms can be accomplished in a single trial by plating on two different media. The resulting counts can provide an understanding of how effective processing is against Salmonella spp. in the base as well as in the inclusion.
For in-plant validation trials, two surrogate organisms which are safe to utilize in the manufacturing environment can be utilized. Organisms such as E. faecium and P. acidilactici have been successfully utilized as surrogates for in plant validation trials. Similar to lab studies, quantification of the two organisms can be accomplished in a single trial by plating on two different media providing valuable information on Salmonella spp. inactivation in both the base formulation as well as the inclusions.
These study options can help you better understand your microbial kill step by providing more information on the microenvironment in your base formulation and inclusions. If you have inclusions in your formulation, consider re-examining your process validation to gain a full understanding of your highest risk ingredients. Even with the purchase of ready to eat inclusions, studies may reveal a need for tighter controls on handling of these materials.

